Biomimicry is a revolutionary concept referring to the imitation of natural processes in artificial settings. It is a hallmark of sustainable research and development (R&D), contributing to circular and regenerative engineering in a host of applications. The concept is simple: We emulate natural processes to resolve critical challenges facing mankind today. Although this approach dovetails nicely with modern sustainability initiatives, it isn’t entirely new. Designers have always turned to nature for inspiration. However, our scientific capabilities have reached a new zenith from which we can apply the smallest – and most successful – of nature’s strategies to some of the largest problems of the day.
Artificial photosynthesis is an exciting example of a biomimetic process. It aims to replicate nature’s most efficient energy conversion process, but instead of converting sunlight, water, and carbon dioxide (CO2) into glucose and oxygen, the goal is to yield hydrogen or other useful chemicals. The process involves two main steps: water splitting and carbon dioxide reduction. Catalysts play a crucial role in both steps, speeding up the reactions and making them more efficient.
The Role of Catalysts in Artificial Photosynthesis
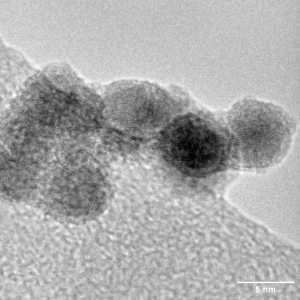
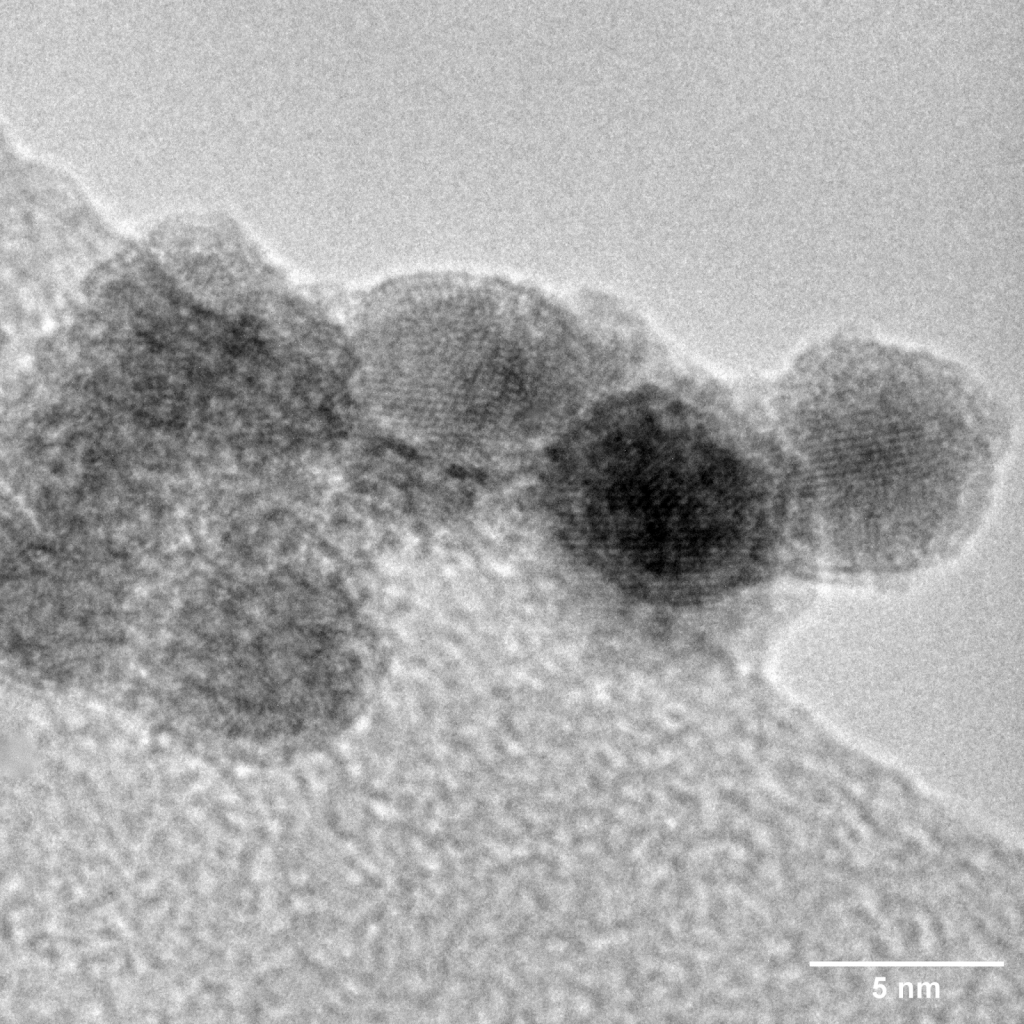
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. In artificial photosynthesis, catalysts are used in both the water splitting and carbon dioxide reduction steps. They help to lower the energy barrier for these reactions, making them proceed more quickly and efficiently. One of the most effective catalysts for the oxygen evolution reaction (OER) in water splitting is iridium. Iridium catalysts have shown excellent performance in terms of activity and stability, yet it remains one of the rarest elements on earth. The scarcity of iridium makes it inordinately expensive and unsustainable for practical, large-scale application.
The Need for Iridium Alternatives
Given the scarcity and high cost of iridium, researchers are actively seeking alternatives. One promising avenue of research is the development of high-entropy alloys (HEAs). These are alloys that contain five or more elements in roughly equal proportions. HEAs have shown promise in a variety of applications, including as catalysts for water splitting.
High-Entropy Alloys: A New Frontier in Water Splitting
High-entropy alloys offer several advantages over traditional catalysts. They are highly stable and resistant to corrosion, making them ideal for long-term use in water splitting reactions. By carefully selecting the ratios of alloying elements, researchers can fine-tune catalytic activity to optimise performance.
Practical Applications of Artificial Photosynthesis Catalysts
The development of efficient and sustainable catalysts for artificial photosynthesis has far-reaching implications. It could lead to the production of clean, renewable hydrogen fuel, helping to reduce our reliance on fossil fuels. Moreover, it could enable the conversion of carbon dioxide, a greenhouse gas, into useful chemicals, helping to mitigate climate change.
Our Contribution to Catalyst Research
At Nikalyte, we are at the forefront of catalyst research. Our NL50 benchtop system allows for the deposition of ultra-pure metallic nanoparticles, including those used in catalysts for artificial photosynthesis. The NL50 utilises magnetron sputtering to generate a beam of ultra-pure nanoparticles in vacuum, which can be generated from any metal or conductive material, including Au, Ag, Cu, Pt, Ir, Ni, Ti, and Zr. This technology opens up new possibilities for the development of high-performance catalysts for artificial photosynthesis.