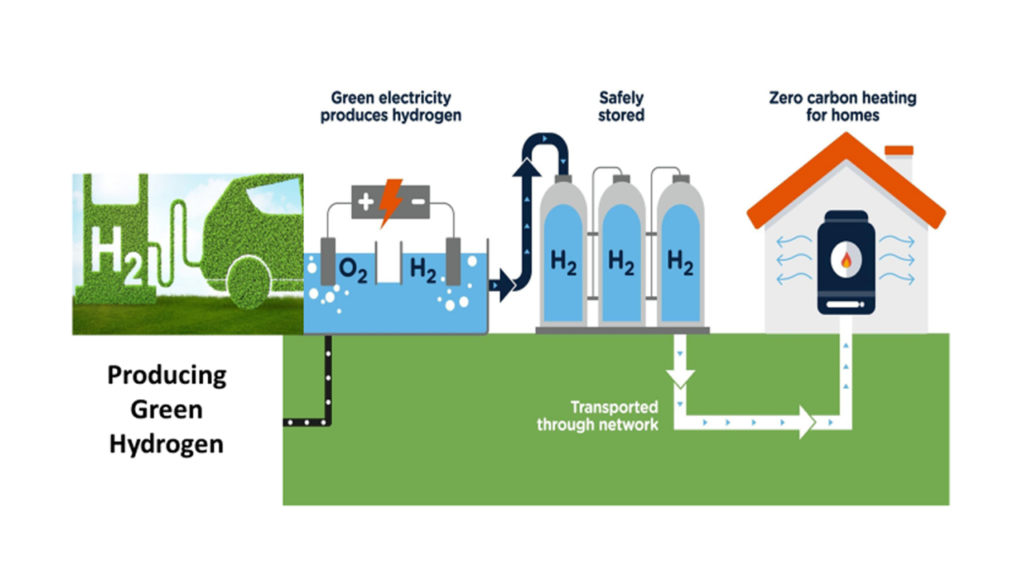
Adopting sustainable energy is crucial for achieving a better future—one that optimizes resource utilization and minimizes the negative impacts of fossil fuel consumption. Green hydrogen, a clean energy source that only emits water vapor as a byproduct, is a promising alternative produced through sustainable energy. The production process involves electrolysis, which separates hydrogen (H2) and oxygen (O2) from water, allowing the hydrogen to be harnessed for energy storage or electricity generation. By leveraging green hydrogen as a power source, we can reduce our dependence on non-renewable fuels while decarbonizing hard-to-abate sectors such as long-haul transportation and heavy industry. Nickel (Ni) catalysts, with their enhanced reactivity and suitability for water electrolysis, have the potential to revolutionize green hydrogen production. Continue reading to discover more insights about the potential of Ni catalysts in this context.
The Need for Catalysts in Effective Electrolysis
Electrolysis involves two primary reactions:
- The Hydrogen Evolution Reaction (HER) at the cathode
- The Oxygen Evolution Reaction (OER) at the anode.
Both reactions require efficient catalysts to lower the activation energy and increase reaction rates. Effective catalysts are essential for achieving high efficiency and productivity in water electrolysis systems. Without catalysts, the overpotentials for HER and OER would be prohibitively high, leading to significant energy losses and making the process economically unfeasible. Therefore, identifying and developing robust, cost-effective, and high-performance catalysts is paramount for advancing green hydrogen production technologies.
The Promise of Nickel as a Catalyst
Ni catalysts have emerged as promising candidates for green hydrogen production via water electrolysis because of several compelling reasons:
High Catalytic Activity for the Hydrogen Evolution Reaction (HER)
Nickel-based materials such as Ni5P4, Ni2Si, and Ni/Y2O3 have demonstrated exceptional electrocatalytic activity for the HER. These materials exhibit catalytic performances that rival, and in some cases, surpass those of precious metal catalysts like platinum. The superior activity of Ni catalysts can be attributed to their optimal hydrogen adsorption energy and efficient water dissociation kinetics. For instance, studies have shown that Ni5P4 exhibits remarkable HER activity. This is due to its favorable electronic structure and synergistic effects between nickel and phosphorus atoms.
Low Cost and Earth Abundance
Nickel is an abundant transition metal. Therefore it is a cost-effective alternative to noble metals, like platinum and iridium, which are scarce and expensive. The widespread availability and low cost of nickel make it a practical choice for large-scale green hydrogen production. This economic advantage is crucial for the widespread adoption and scalability of green hydrogen technologies. Consequently, Ni catalysts offer a more sustainable and economically viable solution for water electrolysis compared to their precious metal counterparts.
Stability Under Alkaline Conditions
Many Ni catalysts exhibit high stability in alkaline media, which is advantageous for water electrolysis. The OER, which occurs at the anode, is more efficient under alkaline conditions due to the reduced overpotential requirements. Ni catalysts maintain their structural integrity and catalytic activity over extended periods under these conditions. They ensure the long-term operational stability and durability of electrolysis systems. The stability of Ni catalysts is a critical factor in reducing maintenance costs. It can also improve the overall reliability of green hydrogen production systems.
Tunable Electronic Structure
The electronic structure of Ni catalysts can be tailored by alloying or forming compounds with other elements. These elements include phosphorus, silicon, or yttrium. This tunability allows for the optimisation of hydrogen adsorption energy and water dissociation kinetics, leading to enhanced HER catalysis. For example, Ni2Si and Ni/Y2O3 have been shown to exhibit modified electronic properties that improve their catalytic performance. By adjusting the composition and structure of Ni catalysts, researchers can fine-tune their catalytic properties. Thus, Ni catalysts can be used for specific electrolysis conditions.
Nanostructuring and Surface Engineering
Advanced techniques in nanostructuring and surface engineering have further enhanced the activity and stability of Ni catalysts. Nanostructuring introduces strain effects, increases active site density, and prevents degradation by creating unique surface morphologies. Surface engineering methods, such as creating Turing patterns or nanotwinning, can modify the Ni catalyst’s surface properties. This has resulted in the improved catalytic performance of Ni catalysts. These techniques enable the development of Ni catalysts with superior properties. As a result, they are highly effective for green hydrogen production.
Optimising the Performance of Your Ni Catalysts with Technology from Nikalyte
Green hydrogen symbolises the way forward for sustainable energy. It has less impact on our planet, offers a means of holding and transporting energy, and has the capability to be used as a fuel for electric vehicles or turbines. Utilising a Ni catalyst is an excellent choice for generating green hydrogen. Not only is nickel less expensive than other metals, like platinum, when applied to a catalyst, meaning that it is an inexpensive option for creating green hydrogen, but the excellent catalytic activity of Ni catalysts ensures it is effective with the HER. We, Nikalyte, have nanoparticle technology available to support Ni catalysts that form green hydrogen. Ensuring a catalyst contains a functionalised surface can help the catalyst to possess specific characteristics, such as selectivity and reactivity. Our nanoparticle deposition tools, like the Nikalyte NL-UHV, can deliver a functionalised surface and ensure the coatings of nanoparticles contain specific attributes, due to their ability to manage the nanoparticles’ compositions, formations, and sizes. Speak with our specialists now to understand which of our technologies would be best for enhancing your Ni catalyst. That way, when utilised for creating green hydrogen, their catalytic performance can be assured.
References:
- Enabling Catalysts for Green Energy. Loughborough University. https://www.lboro.ac.uk/departments/chemical/research-and-innovation/research-areas/net-zero/case-studies/enabling-catalysts-for-green-energy/. Accessed 6th June 2024.
- Helmholtz Association of German Research Centres. Green hydrogen: Nanostructured nickel silicide shines as a catalyst. Phys.org. https://phys.org/news/2022-08-green-hydrogen-nanostructured-nickel-silicide.html. Published 11th August 2022. Accessed 6th June 2024.
- Assabumrungrat S, Fukuhara C, Koo-Amornpattana W, Ratchahat S, Saconsint S, Sae-tang N, Srifa A. Develoment of high-performance nickel-based catalysts for production of hydrogen and carbon nanotubes from biogas. Science Reports. 2022;12(15195). doi:10.1038/s41598-022-1963-y.
- City University of Hong Kong. Revolutionizing stable and efficient catalysts with Turing structures for hydrogen production. ScienceDaily. https://www.sciencedaily.com/releases/2024/01/240108125722.htm. Published 8th January 2024. Accessed 6th June 2024.